Keto Diet and Chronic Pain
Abstract
Ketogenic diets are very low carbohydrate, high fat, moderate protein diets used to treat medication-resistant epilepsy. Growing evidence suggests that one of the ketogenic diet's main mechanisms of action is reducing inflammation. Here, we examined the diet's effects on experimental inflammatory pain in rodent models. Young adult rats and mice were placed on the ketogenic diet or maintained on control diet. After 3–4 weeks on their respective diets, complete Freund's adjuvant (CFA) was injected in one hindpaw to induce inflammation; the contralateral paw was used as the control. Tactile sensitivity (von Frey) and indicators of spontaneous pain were quantified before and after CFA injection. Ketogenic diet treatment significantly reduced tactile allodynia in both rats and mice, though with a species-specific time course. There was a strong trend to reduced spontaneous pain in rats but not mice. These data suggest that ketogenic diets or other ketogenic treatments might be useful treatments for conditions involving inflammatory pain.
Introduction
Acute inflammation is a process in which the innate immune system reacts to tissue infection, irritation, or damage to destroy the infecting pathogen, remove the irritating agent and begin repair of tissue damage. As such, it is a normal and beneficial process. If inflammation is not resolved, however, it will become a chronic state in which healthy tissue is harmed. Chronic inflammation is intertwined inexorably with chronic oxidative stress and elevated levels of free radicals and reactive oxygen species1. Oxidative stress is a key player in many chronic inflammation-related dysfunctions peripherally2,3,4,5 and centrally6,7,8,9. Notably, pain is a major symptom in many inflammation-related disorders, including diabetic neuropathy, chemotherapeutic neuropathy, gout, rheumatoid arthritis, inflammatory bowel disease, and fibromyalgia. Often the pain takes the form of allodynia, a state in which innocuous stimuli are perceived as painful.
Prior studies have shown that diet can influence inflammatory pain, e.g.10,11,12. There is growing evidence that the ketogenic diet (KD) is anti-inflammatory. This diet was introduced as an anticonvulsant treatment for drug-refractory epilepsy, and has very low carbohydrate content as a means to induce a metabolic state similar to fasting but without caloric restriction. In this metabolic state, the liver converts fatty acids to ketone bodies which circulate to be used by other tissues as fuel during reduced availability of glucose; a ketone body-based metabolism should produce fewer free radicals than one based on glucose13. The KD should reduce inflammation as it enhances various antioxidant mechanisms14,15,16,17,18,19,20,21,22,23,24,25,26. Indeed, KD treatment reduces reactive oxygen species17,18,27,28,29,30,31,32, limits oxidative damage to DNA17,20,33, lipids29,34,35,36,37, and proteins16,20,23,35, and modulates immune cell function28,38. Most of these studies did not use a calorie-restricted KD. In spite of the conceptual overlap among inflammation, pain, oxidative stress, and the KD, there has been very little work on the KD as a treatment for inflammatory pain. Here we tested a KD in a rodent model of experimental inflammatory pain, specifically investigating allodynia and measures of spontaneous pain.
Methods
All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee of Trinity College (A3869-01). Male Sprague–Dawley rats and male C57Bl/6 mice were bred in-house (original stock from Charles River), and pair-housed (rats) or group-housed (mice) in 12 h:12 h light:dark conditions. Treatment started at 10–16 week of age (rats) or 6–8 week (mice). Animals remained on their control diet (CD; LabDiet 5001) or were switched to a KD (BioServ 3666); all diets were provided ad libitum. KD was replaced daily. Diet treatment proceeded for three or four weeks before behavioral studies began, and continued through the end of experimentation. Animals were gently handled daily for several days before testing, to reduce handling stress during behavioral studies and to make animals docile for paw volume testing. All testing occurred during the lights-on period of the daily cycle. For rats, 100 µl complete Freund's adjuvant (CFA; Thermo Scientific, 0.5 mg/ml) was injected intraplantar into the right hindpaw with the needle tip as close to the center of the footpads as possible; for mice, injections were similar apart from reducing the dose of CFA to 20 μl. All CFA injections were performed in the morning to keep the four h time point well within the light cycle. Volumes of rat right hindpaws before CFA injection did not differ between CD- and KD-fed groups (p > 0.50), such that the 100 µl dose of CFA can be considered equivalent in the two groups.
Before tactile sensitivity testing, rats were habituated by being brought in their home cage into the testing room for 15 min, then placed on an elevated mesh stand in 20 × 20 × 12 cm acrylic enclosures (to minimize locomotion; IITC) for another 15 min; mice were habituated by being placed on the mesh stand in 10 × 10 × 12 cm acrylic enclosures (IITC) for 60 min. At various times before and after CFA injection, tactile sensitivity was measured with an electronic von Frey probe (IITC, Fig. 1). The rigid von Frey probe was applied alternately to each hindpaw until the animal either withdrew the paw or allowed it to be lifted by the probe; the maximum force on each trial was recorded. Three trials with no less than a 120 s intertrial interval occurred per hindpaw. If the animal began to take a step or otherwise shift its position during a trial, that trial was repeated.
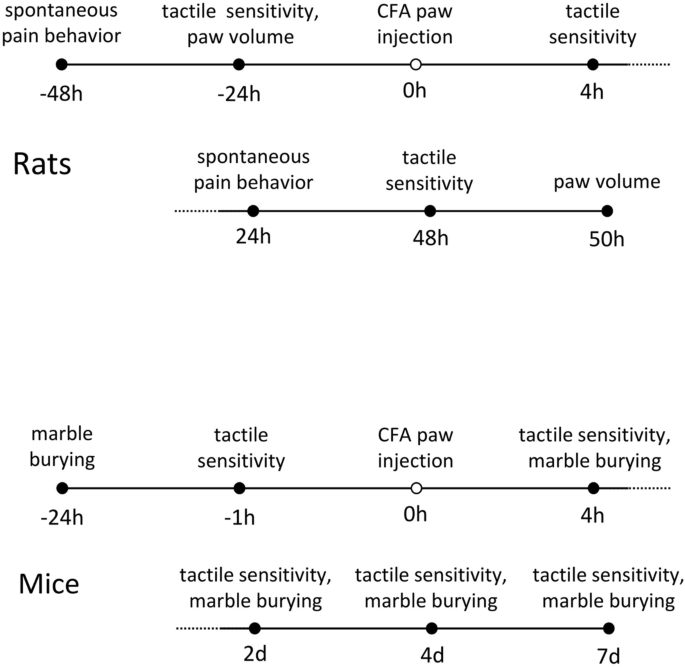
Timeline of experimental procedures in rats and mice.
Full size image
In rats, spontaneous pain behavior was assessed 48 h prior to and 24 h after CFA injection. After a 15 min habituation to the testing room, animals were placed singly in 19 × 29 × 13 cm clear-bottom cages for 30 min and filmed vertically from below. Later, videos were watched for indications of spontaneous pain for 60 s every five minutes; videos were watched by two scorers who were independent and blind to dietary treatment and pre- versus post-CFA state. Epochs with grooming or locomotion were avoided. Position of each hindpaw was placed into the following categories: 0, normal weight bearing; 1, light weight bearing; 2, only paw edge touching floor; 3, paw nearly raised off floor; 4, paw completely raised; 5, licking raised paw, and time spent in each position was recorded. For each observed minute, a weighted pain score was calculated (t1 + 2t2 + … 5t5, in which tx is the time spent in category x) and averaged across the six observed minutes39. This test was not used in mice as preliminary experiments indicated that mouse paws were too small to reliably distinguish the categories.
In rodents, ongoing painful states interfere with ongoing behavior40,41,42. We assessed this effect in mice with marble burying43,44,45 at the indicated times (Fig. 1). Animals were habituated to the testing room for 30 min, then placed individually in 19 × 29 × 13 cm cages for 30 min. These cages had a 3 × 5 array of 1.6 cm diameter black marbles placed on five cm of wood ship bedding. Photos were taken vertically from above before and after the session and analyzed with Photoshop. The quick selection tool was used to measure the total number of pixels associated with the black marbles, and the polygonal lasso tool was used to measure the number of pixels of the bedding field; the number of marble pixels was expressed as a percentage of the entire field's pixels. This calculation was performed on before and after pictures, with the comparative decrease in the marble percentage compared to before indicating the amount of burying. Data from two mice that demonstrated very little burying in the baseline test were excluded from analysis. The marble burying test was not used in rats as preliminary experiments indicated that rats did virtually no burying under our conditions.
Volumes of rat hindpaws were measured by water volume displacement in 25 ml graduated cylinders 24 h prior to and 50 h after CFA injection. At sacrifice of subjects, glucose and the ketone body β-hydroxybutyrate were measured in tail vein blood with Precision Xtra meters (Abbott).
T-tests were used for CD versus KD comparisons, with significance indicated by pound signs. For comparisons of post-CFA time points to baseline time points, multiple Bonferroni comparisons were made, with significance indicated by asterisks. Comparisons were considered significant if p < 0.05. All data are presented as mean ± standard error.
Results
Plantar tactile sensitivity was low in baseline and not different between CD- and KD-fed rats in injected paws (CD 85.0 ± 7.0 g, KD 85.4 ± 10.6 g, p > 0.50) or uninjected paws (CD 95.6 ± 7.3 g, KD 80.8 ± 9.2 g, g > 0.20). All rats demonstrated strong allodynia of the injected hindpaw; however the magnitude of this effect was significantly smaller at 4 h post-injection in KD-fed animals (Fig. 2). This difference did not remain significant at the 48 h time point (Fig. 2). As expected, plantar tactile sensitivity did not change with diet or time in the uninjected hindpaw (Fig. 2).
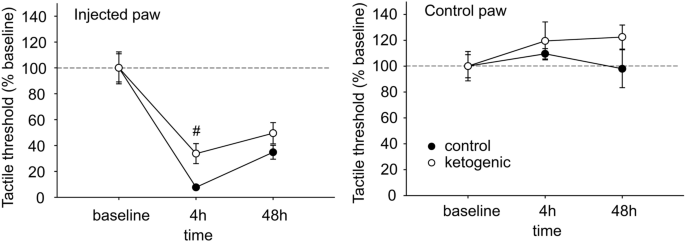
Effects of CFA and diet treatment on tactile allodynia in rats assessed by electronic von Frey probe. For the injected paw, all 4 h and 48 h groups are significantly different from baseline (not indicated). There were no treatment effects on control paw sensitivity. Control n = 14, ketogenic n = 12. #p < 0.05 control v. ketogenic diet.
Full size image
Behaviors indicative of ongoing pain states related to the hindpaws such as licking and avoiding weight bearing were essentially absent pre-CFA, as expected (Fig. 3). After injection, such behaviors were present and directed to only the injected hindpaw in all rats; however there was a strong trend toward less such behavior in KD-fed rats (Fig. 3).
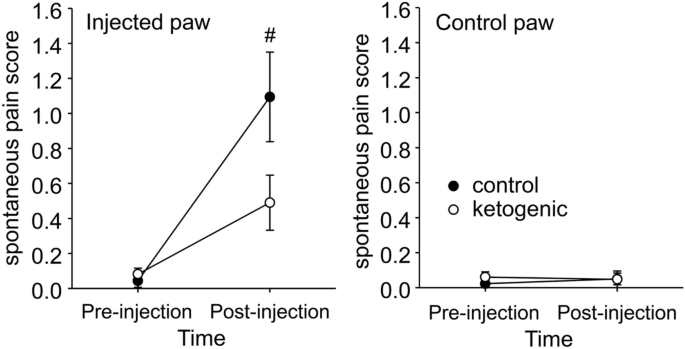
Effects of CFA and diet treatment on spontaneous expression of pain in rats. The spontaneous pain score was calculated from observations on paw favoring, lifting, and licking before and 24 h after CFA injection. For the injected paw, spontaneous pain was significantly higher compared to pre-injection for both groups (not indicated). However, there was a strong trend for spontaneous pain to be lower in ketogenic diet-treated rats. There were no effects regarding the uninjected paw. Control n = 14, ketogenic n = 12. #p = 0.059.
Full size image
As expected46, CFA-induced hindpaw swelling was significantly reduced by KD treatment, measured by change in volume from baseline (Fig. 4). The uninjected hindpaw was unaffected (Fig. 4).
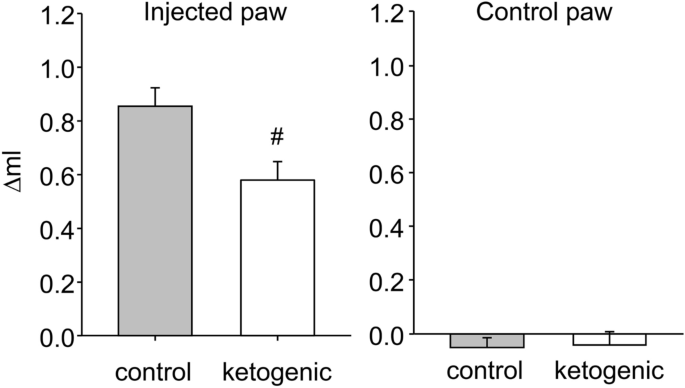
Effects of dietary treatment on CFA-induced inflammatory paw swelling in rats. Paw volume was expressed as the difference between volume at 50 h after CFA injection and volume at baseline. There was significantly less swelling in rats fed the ketogenic diet. There was no effect in control paws. Control n = 14, ketogenic n = 10. #p < 0.05.
Full size image
Plantar tactile sensitivity was low in baseline and not different between CD- and KD-fed mice in injected paws (CD 3.18 ± 0.63 g, KD 2.72 ± 0.47 g, p > 0.50) or uninjected paws (CD 2.84 ± 0.15 g, KD 2.46 ± 0.32 g, g > 0.30).). All mice demonstrated robust tactile allodynia of the CFA-injected hindpaw; however, in CD-fed mice significant allodynia remained out to the last time point assessed (7d), whereas in KD-fed mice allodynia was starting to reverse at 2d and tactile sensitivity was no longer different from baseline at 4d (Fig. 5). As expected, plantar tactile sensitivity did not change with diet or time in the uninjected hindpaw (Fig. 5).
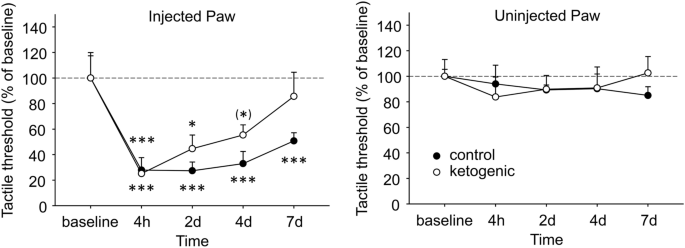
Effects of CFA and diet treatment on tactile allodynia in mouse assessed by electronic von Frey probe. Injected paws became hypersensitive after CFA injection, but the rate of recovery differed in the groups. Control diet-fed mice were still strongly hypersensitive at the last examined timepoint, whereas a gradual and complete recovery occurred in ketogenic diet-fed mice. There were no effects in the uninjected paw. Control n = 6, ketogenic n = 8. ***p < 0.001, **p < 0.05, (*)p = 0.071 compared to baseline.
Full size image
Baseline marble burying was not different between CD- and KD-fed mice (CD 45.2 ± 9.5% buried, KD 32.3 ± 4.7% buried, p > 0.20). Marble burying in mice was strikingly reduced after CFA injection, likely indicating an ongoing pain state (Fig. 6). Burying behavior slowly recovered in both diet groups; and statistics indicated that there was no diet-related difference in recovery rate. Notably, at 4 and 7 days the KD group is burying at levels above baseline, albeit non-significantly, something not found in the CD group (Fig. 6).
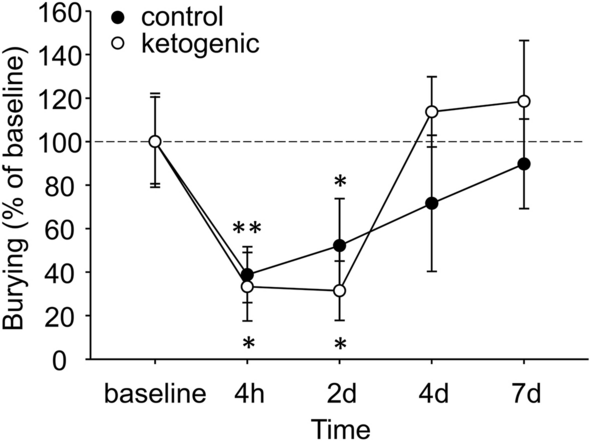
Effects of ongoing pain and dietary treatment on marble burying in mice. CFA injection reduced marble burying in all mice, with no effect of dietary treatment. Control n = 5, ketogenic n = 7. **p < 0.01, *p < 0.05 compared to baseline.
Full size image
Ketogenic diet treatment strongly elevated blood ketone bodies in both species, reduced blood glucose in mice, and produced a trend for decreased mouse body mass (Table 1).
Full size table
Discussion
We found that treatment with a KD significantly ameliorated CFA-induced tactile allodynia in two model species, with more modest effects on indices of ongoing spontaneous pain. A KD-induced amelioration of induced inflammatory pain in rodents is consonant with the KD's reduction of inflammation itself produced by various insults in various tissues in rodents19,28,31,46,47,48,49,50,51,52,53 and patients54,55,56, and reducing pro-inflammatory cytokines19,28,37,47,49,57,58,59,60 and eicosanoids55,58 while elevating anti-inflammatory cytokines60,61. These results may appear to contradict a large body of literature showing that high-fat diets promote inflammation62. However, this literature refers to diets such as the Western or standard American diet (SAD), high in fat but not low in carbohydrates. The metabolic response to dietary fats differs greatly depending on the presence of carbohydrates: the high‐fat‐plus‐carbohydrate diet promotes fat storage, whereas the high fat, low‐carbohydrate diet promotes fat metabolism63. Recently, KD feeding was shown to reverse the tactile allodynia produced in a mouse model of metabolic syndrome64 (strikingly, the KD in this study had almost twice the fat level of the high-fat/moderate carbohydrate diet that induced metabolic syndrome). Metabolic syndrome-related allodynia relates to inflammation in the peripheral nervous system65, and as diabetic neuropathy is thought also to involve inflammation in the spinal cord66, beneficial effects of the KD against pain syndromes involving inflammation could be peripheral, central, or both. KD treatment, however, appears not to be equally effective in all neuropathic pain syndromes67 possibly relating to involvement of inflammation.
Clinical work with KD and pain had a very early start, specifically regarding migraine68,69 and this use may be undergoing a resurgence70. Notably, oxidative stress has been hypothesized to be the trigger of many types of migraines71. KD feeding effectively treats pain and other symptoms in inflammatory bowel syndrome72 and Parkinson's disease73. Overall body pain is alleviated in overweight diabetic patients74, although it was not specified if the type of pain was neuropathic. Given the metabolic parallels between KD treatment and fasting, and the established efficacy of fasting against rheumatoid arthritis75,76, a KD could be particularly useful in this disorder. Existing studies suggest little clinical benefit77,78; however, KD treatment in these studies lasted only seven days (to parallel a fasting treatment, which was itself effective). We have found that antinociceptive effects of the KD evolve over days to weeks79 and others have found a similar pattern in reduced oxidative stress18, suggesting that longer treatment durations should be attempted in rheumatoid arthritis.
There were several differences between the results with rats and mice. Mice appeared to be more affected physiologically by ketogenic diet treatment, with lowered glucose and a trend to lower body mass (not occurring in rats) and a more than two-fold higher elevation in β-hydroxybutyrate than in rats. These are clearly species related. There were also differences in behavioral outcomes. The ketogenic diet improved tactile allodynia in rats at four hour post-injection, but not later, whereas beneficial effects on tactile allodynia in mice appeared in the two to four day range and persisted thereafter. Given relative species paw sizes and the currently used doses of CFA, the effective dose in mice is substantially higher, and so either or both dose and species could underlie these differences in diet responsiveness. Ongoing pain states were unaffected by diet in mice, with a trend to improvement in rats. Species differences, however, led us to use different tests for each species. The mouse marble burying test clearly showed that a state of ongoing pain reduced performance of this behavior. The lack of a ketogenic diet effect indicates that either that the diet does not improve spontaneous pain in this model in mice, or that marble burying is inappropriate behavior to assess these types of changes.
It is somewhat unclear which of the main metabolic actions of a KD (elevated ketone bodies, lowered and less variable glucose) leads to limiting inflammation and inflammatory pain. Certainly, chronically elevated glucose is undesirable: high fasting glucose and/or impaired glucose tolerance associate with elevated blood cytokines80,81,82,83,84,85, C-reactive protein80,81,82,86, oxidative markers87, circulating white blood cells88, and inflammatory response of white blood cells89. In fact, acute hyperglycemia elevates circulating cytokines through an oxidative mechanism90. On the other hand, elevated ketone bodies themselves seem to have beneficial effects: in vivo and in vitro studies show that β-hydroxybutyrate itself moderates the endoplasmic reticulum stress-induced inflammasome91,92 and the NRLP3 inflammasome49,60,92,93,94 in various organs in a manner apparently unrelated to its use as a substrate for the tricarboxylic acid cycle93. In addition, free fatty acids from a KD might be directly beneficial by reducing mitochondrial production of reactive oxygen species27. Besides being anti-inflammatory, there are other possible mechanisms for a KD to limit pain95. Regardless of mechanism, this study and a growing body of evidence suggest that pain be added as a variable in more clinical studies of the KD generally, and specifically that more studies of KD treatment in clinical inflammatory pain syndromes is warranted.
References
- 1.
Lugrin, J. et al. The role of oxidative stress during inflammatory processes. Biol. Chem. 395(2), 203–230 (2014).
CAS PubMed Article Google Scholar
- 2.
El Assar, M., Angulo, J. & Rodriguez-Manas, L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic. Biol. Med. 149, 72–77 (2020).
PubMed Article CAS Google Scholar
- 3.
Newsholme, P. et al. Oxidative stress pathways in pancreatic beta-cells and insulin-sensitive cells and tissues: Importance to cell metabolism, function, and dysfunction. Am. J. Physiol. Cell Physiol. 317(3), C420–C433 (2019).
CAS PubMed Article Google Scholar
- 4.
Dinh, Q. N. et al. Pressor response to angiotensin II is enhanced in aged mice and associated with inflammation, vasoconstriction and oxidative stress. Aging (Albany NY) 9(6), 1595–1606 (2017).
CAS Article Google Scholar
- 5.
Oyarce, M. P. & Iturriaga, R. Contribution of oxidative stress and inflammation to the neurogenic hypertension induced by intermittent hypoxia. Front. Physiol. 9, 893 (2018).
PubMed PubMed Central Article Google Scholar
- 6.
Pangrazzi, L., Balasco, L., & Bozzi, Y. Oxidative stress and immune system dysfunction in autism spectrum disorders. Int. J. Mol. Sci. 21(9) (2020).
- 7.
Puttachary, S. et al. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed. Res. Int. 2015, 745613 (2015).
PubMed PubMed Central Article CAS Google Scholar
- 8.
Blesa, J. et al. Oxidative stress and Parkinson's disease. Front. Neuroanat. 9, 91 (2015).
PubMed PubMed Central Google Scholar
- 9.
Abdul-Muneer, P. M., Chandra, N. & Haorah, J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 51(3), 966–979 (2015).
CAS PubMed Article Google Scholar
- 10.
Totsch, S. K. et al. Effects of a Standard American Diet and an anti-inflammatory diet in male and female mice. Eur. J. Pain 22(7), 1203–1213 (2018).
CAS PubMed Article Google Scholar
- 11.
Totsch, S. K. et al. The impact of the Standard American Diet in rats: Effects on behavior, physiology and recovery from inflammatory injury. Scand. J. Pain 17, 316–324 (2017).
PubMed Article Google Scholar
- 12.
Totsch, S. K. et al. Total Western diet alters mechanical and thermal sensitivity and prolongs hypersensitivity following complete Freund's adjuvant in mice. J. Pain 17(1), 119–125 (2016).
PubMed Article Google Scholar
- 13.
Veech, R. L. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids 70, 309–319 (2004).
CAS PubMed Article Google Scholar
- 14.
Kim, D. Y. et al. Ketones prevent oxidative impairment of hippocampal synaptic integrity through KATP channels. PLoS ONE 10, e0119316 (2015).
PubMed PubMed Central Article CAS Google Scholar
- 15.
Nazarewicz, R. R. et al. Effect of short-term ketogenic diet on redox status of human blood. Rejuvenation Res. 10, 435–439 (2007).
CAS PubMed Article Google Scholar
- 16.
Greco, T. et al. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J. Cereb. Blood Flow Metab. 36, 1603–1613 (2016).
CAS PubMed Article Google Scholar
- 17.
Jarrett, S. G. et al. The ketogenic diet increases mitochondrial glutathione levels. J. Neurochem. 106, 1044–1051 (2008).
CAS PubMed Article Google Scholar
- 18.
Milder, J. B., Liang, L.-P. & Patel, M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol. Dis. 40, 238–244 (2010).
CAS PubMed PubMed Central Article Google Scholar
- 19.
Lu, Y. et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci. Lett. 683, 13–18 (2018).
CAS PubMed Article Google Scholar
- 20.
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
ADS CAS Article Google Scholar
- 21.
Elamin, M. et al. Ketone-based metabolic therapy: Is increased NAD+ a primary mechanism?. Front. Mol. Neurosci. 10, 377 (2017).
PubMed PubMed Central Article CAS Google Scholar
- 22.
Scheibye-Knudsen, M. et al. A high-fat diet and NAD+ activate Sirt1 to rescue premature aging in Cockayne syndrome. Cell. Metab. 20(5), 840–855 (2014).
CAS PubMed PubMed Central Article Google Scholar
- 23.
Kephart, W.C., et al. The 1-week and 8-month effects of a ketogenic diet or ketone salt supplementation on multi-organ markers of oxidative stress and mitochondrial function in rats. Nutrients 9, E1019 (2017).
- 24.
Knowles, S. et al. Ketogenic diet regulates the antioxidant catalase via the transcription factor PPARγ2. Epilepsy Res. 147, 71–74 (2018).
CAS PubMed PubMed Central Article Google Scholar
- 25.
Benlloch, M. et al. Satiating effect of a ketogenic diet and its impact on muscle improvement and oxidation state in multiple sclerosis patients. Nutrients 11, 1156 (2019).
CAS PubMed Central Article Google Scholar
- 26.
Nakamura, K., et al. A ketogenic formula prevents tumor progression and cancer cachexia by attenuating systemic inflammation in colon 26 tumor-bearing mice. Nutrients 10(2) (2018).
- 27.
Davis, L. M., Rho, J. M. & Sullivan, P. G. UCP-mediated free fatty acid uncoupling of isolated cortical mitochondria from fasted animals: correlations to dietary modulations. Epilepsia 49(Suppl. 8), 117–119 (2008).
PubMed PubMed Central Article Google Scholar
- 28.
Kim, D. Y. et al. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS ONE 7, e35476 (2012).
ADS CAS PubMed PubMed Central Article Google Scholar
- 29.
Kong, G. et al. The ketone metabolite β-hydroxybutyrate attenuates oxidative stress in spinal cord injury by suppression of class I histone deacetylases. J. Neurotrauma 34, 2645–2655 (2017).
PubMed Article Google Scholar
- 30.
Sullivan, P. G. et al. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 55, 576–580 (2004).
CAS PubMed Article Google Scholar
- 31.
Bernardo-Colon, A. et al. Antioxidants prevent inflammation and preserve the optic projection and visual function in experimental neurotrauma. Cell Death Dis. 9(11), 1097 (2018).
PubMed PubMed Central Article CAS Google Scholar
- 32.
Stafford, P. et al. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr. Metab. (Lond.) 7, 74 (2010).
Article CAS Google Scholar
- 33.
Elamin, M. et al. Ketogenic diet modulates NAD+-dependent enzymes and reduces DNA damage in hippocampus. Front. Cell. Neurosci. 12, 263 (2018).
PubMed PubMed Central Article CAS Google Scholar
- 34.
Ni, H., Zhang, D.-J. & Tian, T. Ketogenic diet change cPLA2/clusterin and autophagy related gene expression and correlate with cognitive deficits and hippocampal MFs sprouting following neonatal seizures. Epilepsy Res. 120, 13–18 (2016).
CAS PubMed Article Google Scholar
- 35.
Pawlosky, R. J. et al. Effects of a dietary ketone ester on hippocampal and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer's disease. J. Neurochem. 141, 195–207 (2017).
CAS PubMed PubMed Central Article Google Scholar
- 36.
Rhyu, H.-S., Cho, S.-Y. & Roh, H.-T. The effects of ketogenic diet on oxidative stress and antioxidative capacity markers of Taekwondo athletes. J. Exerc. Rehabil. 10, 362–366 (2014).
PubMed PubMed Central Article Google Scholar
- 37.
Bosco, G. et al. Effects of the ketogenic diet in overweight divers breathing enriched air Nitrox. Sci. Rep. 8, 2655 (2018).
ADS PubMed PubMed Central Article CAS Google Scholar
- 38.
Rahman, M. et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 5, 3944 (2014).
ADS CAS PubMed Article Google Scholar
- 39.
Paulson, P. E., Morrow, T. J. & Casey, K. L. Bilateral behavioral and regional cerebral blood flow changes during painful peripheral mononeuropathy in the rat. Pain 84, 233–245 (2000).
CAS PubMed PubMed Central Article Google Scholar
- 40.
Andrews, N. et al. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur. J. Pain 16, 485–495 (2012).
CAS PubMed Article Google Scholar
- 41.
Leitl, M. D. et al. Sustained pain-related depression of behavior: Effects of intraplantar formalin and complete Freund's adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol. Pain 10, 62 (2014).
PubMed PubMed Central Article Google Scholar
- 42.
Rutten, K. et al. Burrowing as a non-reflex behavioural readout for analgesic action in a rat model of sub-chronic knee joint inflammation. Eur. J. Pain 18(2), 204–212 (2014).
CAS PubMed Article Google Scholar
- 43.
Castro, K. et al. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutr. Neurosci. 20, 343–350 (2017).
CAS PubMed Article Google Scholar
- 44.
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
CAS PubMed PubMed Central Article Google Scholar
- 45.
Kim, S. et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532 (2017).
ADS PubMed PubMed Central Article CAS Google Scholar
- 46.
Ruskin, D. N. et al. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS ONE 4, e8349 (2009).
ADS PubMed PubMed Central Article CAS Google Scholar
- 47.
Dupuis, N. et al. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia 56(7), e95–e98 (2015).
CAS PubMed Article Google Scholar
- 48.
Nandivada, P. et al. Eucaloric ketogenic diet reduces hypoglycemia and inflammation in mice with endotoxemia. Lipids 51, 703–714 (2016).
CAS PubMed Article Google Scholar
- 49.
Goldberg, E. L. et al. β-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 18, 2077–2087 (2017).
CAS PubMed PubMed Central Article Google Scholar
- 50.
Jeong, E. A. et al. Ketogenic diet-induced peroxisome proliferator-activated receptor-γ activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp. Neurol. 232, 195–202 (2011).
CAS PubMed Article Google Scholar
- 51.
Yang, X. & Cheng, B. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J. Mol. Neurosci. 42, 145–153 (2010).
CAS PubMed Article Google Scholar
- 52.
Locker, F. et al. The influence of ketogenic diets on psoriasiform-like skin inflammation. J. Invest. Dermatol. 140(3), 707-710.e7 (2020).
CAS PubMed Article Google Scholar
- 53.
Karagiannis, F. et al. Lipid-droplet formation drives pathogenic group 2 innate lymphoid cells in airway inflammation. Immunity 52(4), 620-634.e6 (2020).
CAS PubMed Article Google Scholar
- 54.
Yarar-Fisher, C. et al. Evaluation of a ketogenic diet for improvement of neurological recovery in individuals with acute spinal cord injury: A pilot, randomized safety and feasibility trial. Spinal Cord Ser. Cases 4, 88 (2018).
PubMed PubMed Central Article Google Scholar
- 55.
Bock, M., Karber, M. & Kuhn, H. Ketogenic diets attenuate cyclooxygenase and lipoxygenase gene expression in multiple sclerosis. EBioMedicine 36, 293–303 (2018).
PubMed PubMed Central Article Google Scholar
- 56.
Phillips, M. C. L. et al. Impact of a ketogenic diet on sporadic inclusion body myositis: A case study. Front. Neurol. 11, 582402 (2020).
PubMed PubMed Central Article Google Scholar
- 57.
Forsythe, C. E. et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 43, 65–77 (2008).
CAS PubMed Article Google Scholar
- 58.
de Luis, D. A. et al. Effect of a hypocaloric diet with a commercial formula in weight loss and quality of life in obese patients with chronic osteoarthritis. Nutr. Hosp. 27, 1648–1654 (2012).
PubMed Google Scholar
- 59.
McKay, A. K. A. et al. Chronic adherence to a ketogenic diet modifies Iron metabolism in elite athletes. Med. Sci. Sports Exerc. 51(3), 548–555 (2019).
CAS PubMed Article Google Scholar
- 60.
Harun-Or-Rashid, M. & Inman, D. M. Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J. Neuroinflamm. 15(1), 313 (2018).
MathSciNet CAS Article Google Scholar
- 61.
Ishimwe, J. A., Garrett, M. R. & Sasser, J. M. 1,3-Butanediol attenuates hypertension and suppresses kidney injury in female rats. Am. J. Physiol. Renal Physiol. 319(1), F106–F114 (2020).
CAS PubMed Article PubMed Central Google Scholar
- 62.
Johnson, A. R. & Makowski, L. Nutrition and metabolic correlates of obesity and inflammation: Clinical considerations. J. Nutr. 145, 1131S-1136S (2015).
CAS PubMed PubMed Central Article Google Scholar
- 63.
Forsythe, C. E. et al. Limited effect of dietary saturated fat on plasma saturated fat in the context of a low carbohydrate diet. Lipids 45, 947–962 (2010).
CAS PubMed PubMed Central Article Google Scholar
- 64.
Cooper, M. A. et al. A ketogenic diet reduces metabolic syndrome-induced allodynia and promotes peripheral nerve growth in mice. Exp. Neurol. 306, 149–157 (2018).
PubMed PubMed Central Article Google Scholar
- 65.
Cooper, M. A. et al. Modulation of diet-induced mechanical allodynia by metabolic parameters and inflammation. J. Peripher. Nerv. Syst. 22, 39–46 (2017).
CAS PubMed PubMed Central Article Google Scholar
- 66.
Wang, D., Couture, R. & Hong, Y. Activated microglia in the spinal cord underlies diabetic neuropathic pain. Eur. J. Pharmacol. 728, 59–66 (2014).
CAS PubMed Article PubMed Central Google Scholar
- 67.
Masino, S. A. & Ruskin, D. N. Ketogenic diets and pain. J. Child Neurol. 28, 993–1001 (2013).
PubMed PubMed Central Article Google Scholar
- 68.
Baborka, C. J. Migraine: results of treatment by ketogenic diet in fifty cases. Mayo Clin. Proc. 5(190–191), 190–191 (1930).
Google Scholar
- 69.
Maggioni, F., Margoni, M. & Zanchin, G. Ketogenic diet in migraine treatment: A brief but ancient history. Cephalalgia 31, 1150–1151 (2011).
PubMed Article PubMed Central Google Scholar
- 70.
Di Lorenzo, C., et al. A randomized double-blind, cross-over trial of very low-calorie diet in overweight migraine patients: A possible role for ketones? Nutrients 11(8) (2019).
- 71.
Borkum, J. M. Migraine triggers and oxidative stress: A narrative review and synthesis. Headache 56, 12–35 (2016).
PubMed Article Google Scholar
- 72.
Austin, G. L. et al. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 7, 706-708.e1 (2009).
CAS PubMed PubMed Central Article Google Scholar
- 73.
Phillips, M. C. L. et al. Low-fat versus ketogenic diet in Parkinson's disease: A pilot randomized controlled trial. Mov. Disord. 33(8), 1306–1314 (2018).
CAS PubMed PubMed Central Article Google Scholar
- 74.
Guldbrand, H. et al. Randomization to a low-carbohydrate diet advice improves health related quality of life compared with a low-fat diet at similar weight-loss in Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 106(2), 221–227 (2014).
CAS PubMed Article Google Scholar
- 75.
Hafström, I. et al. Effects of fasting on disease activity, neutrophil function, fatty acid composition, and leukotriene biosynthesis in patients with rheumatoid arthritis. Arthritis Rheum. 31(5), 585–592 (1988).
PubMed Article Google Scholar
- 76.
Udén, A. M. et al. Neutrophil functions and clinical performance after total fasting in patients with rheumatoid arthritis. Ann. Rheum. Dis. 42(1), 45–51 (1983).
PubMed PubMed Central Article Google Scholar
- 77.
Fraser, D. A. et al. Reduction in serum leptin and IGF-1 but preserved T-lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 18, 209–214 (2000).
CAS PubMed Google Scholar
- 78.
Fraser, D. A. et al. Serum levels of interleukin-6 and dehydroepiandrosterone sulphate in response to either fasting or a ketogenic diet in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 18, 357–362 (2000).
CAS PubMed Google Scholar
- 79.
Ruskin, D. N. et al. Ketogenic diets and thermal pain: Dissociation of hypoalgesia, elevated ketones, and lowered glucose in rats. J. Pain 14, 467–474 (2013).
CAS PubMed PubMed Central Article Google Scholar
- 80.
Zuo, H. et al. Diabetes, impaired fasting glucose and their relations to plasma pro-inflammatory cytokines: a population-based study in China. Diabet. Med. 27, 1461–1463 (2010).
CAS PubMed Article Google Scholar
- 81.
Kuzmicki, M. et al. Circulating pro- and anti-inflammatory cytokines in Polish women with gestational diabetes. Horm. Metab. Res. 40, 556–560 (2008).
CAS PubMed Article Google Scholar
- 82.
Colak, A. et al. Postload hyperglycemia is associated with increased subclinical inflammation in patients with prediabetes. Scand. J. Clin. Lab. Invest. 73, 422–427 (2013).
CAS PubMed Article Google Scholar
- 83.
Butkowski, E. G. & Jelinek, H. F. Hyperglycaemia, oxidative stress and inflammatory markers. Redox Rep. 22, 257–264 (2017).
CAS PubMed Article Google Scholar
- 84.
Kato, K. et al. Association between elevated C-reactive protein levels and prediabetes in adults, particularly impaired glucose tolerance. Can. J. Diabetes 43(1), 40-45.e2 (2019).
PubMed Article Google Scholar
- 85.
Sesti, G. et al. Elevated 1-h post-load plasma glucose levels in subjects with normal glucose tolerance are associated with unfavorable inflammatory profile. Acta Diabetol. 51, 927–932 (2014).
CAS PubMed Article Google Scholar
- 86.
Kato, K., et al. Association between elevated C-reactive protein levels and prediabetes in adults, particularly impaired glucose tolerance. Can. J. Diabetes 43(1), 40–45 e2 (2019).
- 87.
Korkmaz, G. G. et al. Total antioxidant status and markers of oxidative stress in subjects with normal or impaired glucose regulation (IFG, IGT) in diabetic patients. Scand. J. Clin. Lab. Invest. 73, 641–649 (2013).
CAS PubMed Article Google Scholar
- 88.
Jiang, H. et al. Elevated white blood cell count is associated with higher risk of glucose metabolism disorders in middle-aged and elderly Chinese people. Int. J. Environ. Res. Public Health 11, 5497–5509 (2014).
CAS PubMed PubMed Central Article Google Scholar
- 89.
Volpe, C. M. O. et al. The production of nitric oxide, IL-6, and TNF-alpha in palmitate-stimulated PBMNCs is enhanced through hyperglycemia in diabetes. Oxid. Med. Cell. Longevity 2014, 479587 (2014).
Article CAS Google Scholar
- 90.
Esposito, K. et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 106, 2067–2072 (2002).
CAS PubMed Article Google Scholar
- 91.
Bae, H. R. et al. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 7, 66444–66454 (2016).
PubMed PubMed Central Article Google Scholar
- 92.
Guo, M. et al. Ketogenic diet improves brain ischemic tolerance and inhibits NLRP3 inflammasome activation by preventing Drp1-mediated mitochondrial fission and endoplasmic reticulum stress. Front Mol Neurosci 11, 86 (2018).
PubMed PubMed Central Article CAS Google Scholar
- 93.
Youm, Y. H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21(3), 263–269 (2015).
CAS PubMed PubMed Central Article Google Scholar
- 94.
Zhang, N. et al. Amelioration of clinical course and demyelination in the cuprizone mouse model in relation to ketogenic diet. Food Funct. 11(6), 5647–5663 (2020).
CAS PubMed Article Google Scholar
- 95.
Ruskin, D.N. Metabolic therapy and pain. in Ketogenic Diet and Metabolic Therapies: Expanded Roles in Health and Disease pp. 196–208 (Oxford University Press, New York, 2017).
Download references
Acknowledgements
The authors thank Carter F. Jones, Zach X. Yung, and Allison J. Wells for technical assistance. Supported by National Institutes of Health NS066392 (SAM), NS065957 (SAM), AT008742 (DNR).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Ruskin, D.N., Sturdevant, I.C., Wyss, L.S. et al. Ketogenic diet effects on inflammatory allodynia and ongoing pain in rodents. Sci Rep 11, 725 (2021). https://doi.org/10.1038/s41598-020-80727-x
Download citation
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-020-80727-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Source: https://www.nature.com/articles/s41598-020-80727-x